
Finally, calculate yi using Raoults law (Use Antoine Eqn for Pisat)ħ6.4244.221.971776.4376. thinking this is because of vapor cross channeling. Stop if this is equal or close to earlier value of T, else use this value as a new guess.
Calculate ik, (note: Number of ik is equal to number of component)Ħ. The solution is not straightforward as T is unknown.īUBL T calculationSolution is through iteration, XiKi 1 (10.13), For Raoult's Law, Pb xiPisat (10.2) Pb xiPisat Pksat xi Pi So lets calculate Tb at z1=0.6 and P=70kPa.This is point b in previous Tx1y1 diagram. Plot Tx1y1 at P=70kPaChoose T between T1sat and T2sat, calculate P1sat and P2sat and use these to calculate x1 by the following eqn. =1 (10.14) If Raoult's Law valid, Pd 1yiPi So lets calculate Pd at z1=0.6 and T=75oC.This is point c in previous Px1y1 diagram. For Raoult's Law, P xiPisat (10.2) P x1P1sat x2P2sat x1P1sat (1 x1)P2satP (P1sat P2sat )x1 P2sat note: a linear line (y=mx+c)Ĭalculate P for a set of x1 and then calculate y1using,ġ83.211750.888074.960.8750.748366.720.6750.569258.470.4750.331350.230.275041.98075y1P(kPa)x1Tī sat liquid solution or bubblepointPb is by BUBL PĬ sat vapor mixtureor dewpointPd is by DEW PĭEW P calculationCalculate Pd and x1, given y1 and T Substitute yi Kixi into (A)zi (1V )xi KixiV xi (1V VKi ) xi (1V (Ki 1))xiĪcetonitrile(1)/Nitromethane(2)Antoine Eqn,Ĭalculate P and y1, given a set of x1 and T=75oCThis is BUBL P calculation. Let T=1 mol, so V and L are mole fractionszi Lxi Vyi zi (1V )xi Vyi (A) To calculate the T when the last dew disappear as a result of increase in T at constant P. To calculate the T when the 1st dew (drop of liquid) appear as a result of decrease in T at constant P Read value of K-valueat given T and Pe.g.

Top surface issaturated-liquid(P,T,x1 surface)ĪEDBLA givesFigure 10.2 (a)Pxy phase diagram at constant T Under surface issaturated-vapor (P,T,y1 surface) T curve for species 2Ĭ1 and C2 are critical points for species 1 & 2 Carry out bubble and dew point calculations for a given mixture Carry out flash calculation in order to determine the vapor/liquidįraction as well as the mixture composition of each phase at specified conditions using available K-Values etc. Apply Raoults law and Henrys law to solve simple thermodynamic Apply simplified VLE equations to obtain data for P-XY, T-XY and X.

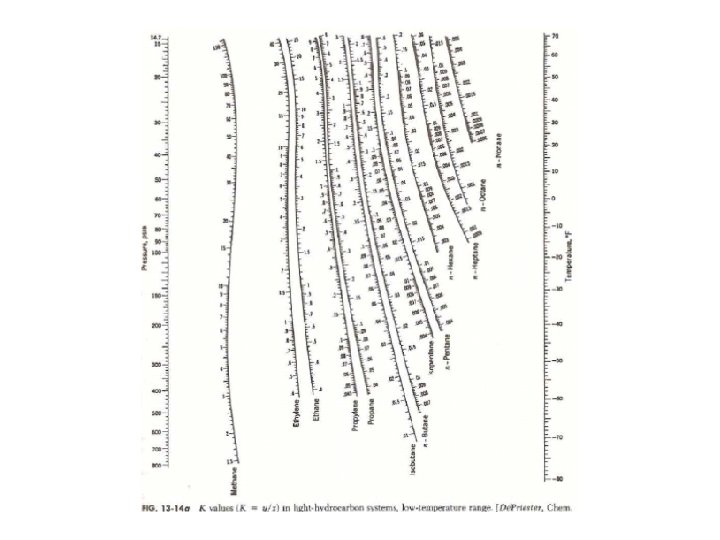
DEPRIESTER CHART ETHANOL HOW TO
It is expected that students have the ability to: Describe the behaviour of VLE and how to simplify the VLE problem. SKF 2213 Chemical Engineering Thermodynamics


 0 kommentar(er)
0 kommentar(er)
